Site Navigation
Oilfield expertise at your desktop
Pyrolysis
“The process of thermochemical decay of organic material at high temperature without oxygen is known as Pyrolysis”. Pyrolysis is mainly used to measure maturity and richness of possible source rocks by the Organic geochemists. During pyrolysis analysis, organic content in source rock is pyrolysed without any oxygen which is followed by the process of combustion. This is followed by the measurement of the hydrocarbon content and amount of released carbon dioxide . “Rock-Eval” is considered as one of the most commonly used pyrolysis techniques.During this pyrolysis technique, an sample is kept within a vessel followed by progressive heating to 550 degC in an inert surrounding. At a moderate temperature, the hydrocarbons found in samples are volatilized. The hydrocarbon quantity is than measured and registered as S1 peak.
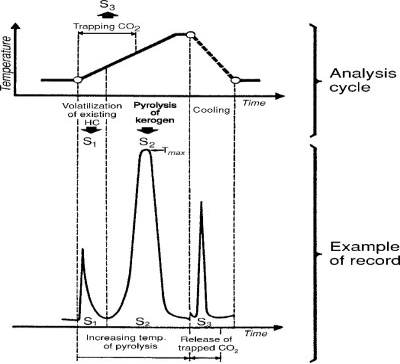
Kerogen available in sample, is next pyrolysed and generates hydrocarbons as well as other compounds resembling hydrocarbons ( registered as S2 peak ) , CO2 and water . The generated CO2 is registered as S3 peak, whereas remaining carbon is registered as S4. The diagram below presents analysis and corresponding recording cycles (Figure 1).
| Peak | Measurement provides | Remarks |
| “S1 mg Hc/g” |
The movable hydrocarbon available in sample initially. | Can be assumed to be remaining hydrocarbon. When S1 peak is high compared to S2, an secondary source like hydrocarbon contamination or migration could be possible. |
| “S2 mg Hc/g” |
The amount of hydrocarbons formed by thermal pyrolysis . | Used to estimate the remaining hydrocarbon generating potential of the sample |
| “S3 mg CO2/g” |
The CO2 produced by kerogen’s thermal breakdown. | This happens a lot in source rocks which are calcareous in nature. |
| “S4 mg carbon/g” |
The remaining carbon content | Remaining carbon content in sample has almost zero potential to yield hydrocarbons because of improper chemical structure and unavailability of hydrogen . |

Fig-1 Cycle of analysis and corresponding recording from Tissot,B.P and Weite ,D H 1984.
Finally Percent TOC is calculated using the below formula.
%TOC = [0.082(S1+S2)+S4]/10 milligrams hydrocarbon per gram of rock (weight percent organic carbon divided by dry rock weight).